Prevulcanization Inhibitors for Sulfur Vulcanization
To avoid premature vulcanization during processing, retarders are often added to the cure system. These compounds increase the induction time by reacting with the active accelerator fragment which slows down or temporarily prevents zinc salt formation.
Retarders, which are also called pre-vulcanization inhibitors (PVI), are often compounds that readily react with the accelerator (fragments) and only slowly release them. To function as a retarder, the associated polysulfides should also not readily react with the polydiene to produce sulfur bridges. A compound that meets both requirements is N-(cyclohexylthio) phthalimide (CTP). It has been shown that CTP reacts rapidly with thiazoles to form CDB and phthalamide as shown below.1,2 In the case of benzothiazoles, it has been postulated that CTP reacts faster with 2-mercaptobenzothiazole (MBT), the active accelerator fragment, than MBT reacts with the primary accelerator to form 2-2'-dithiobis(benzothiazole) (MBTS).7 Since CDB only slowly reverts back to thiazoles, the formation of MBTS with subsequent cross-linking is significantly delayed, or in other words the reaction of CTP with MBT is responsible for the retardation action of CTP. The source of MBT is either the decomposition of MBS or the cross-linking step which releases MBT as a byproduct.
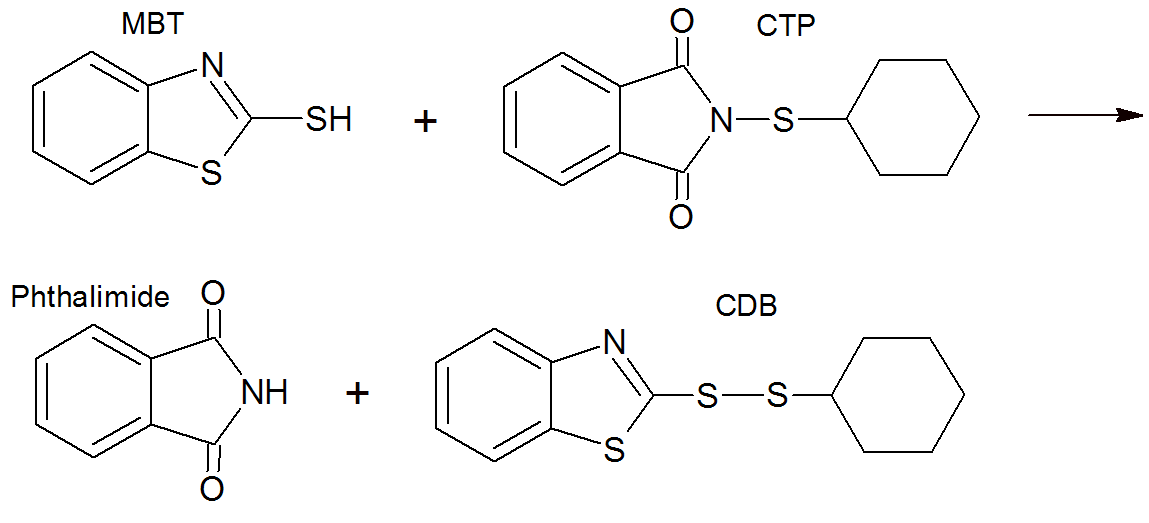
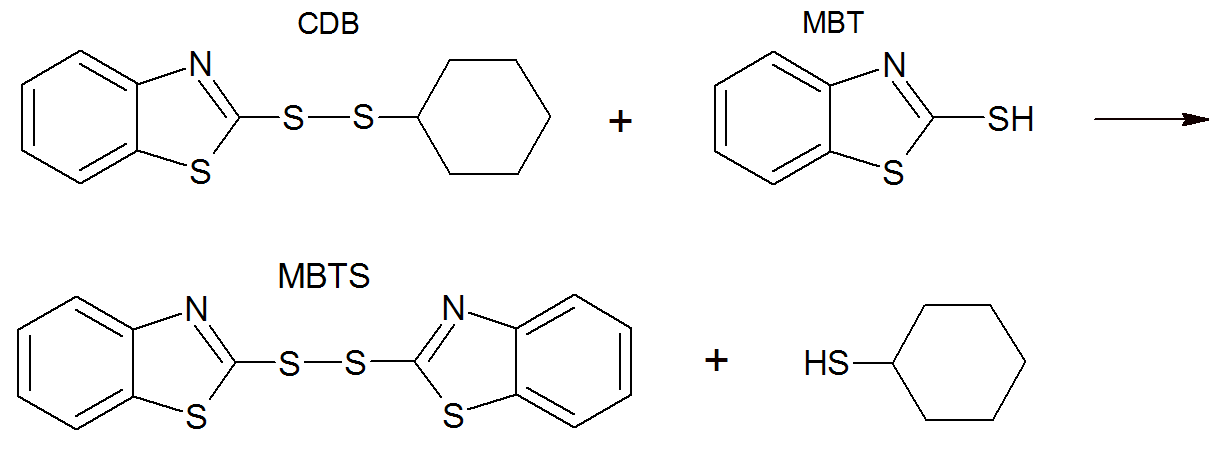
The CDB slowly converts back into the vulcanization system, for example by reacting with more MBT to yield MBTS (see below) which then undergoes reactions which result in the formation of crosslinks.3,4
Despite its strong retardation effect, CTP has little effect on the actual cure rate once cure is initiated and all CTP is consumed. Furthermore, CTP does not (much) affect the degree of cure, the maximum cross-link density, and length of sulfur bridges. CTP has also the advantage that it does not cause staining and that it permits processing at higher temperatures which increases productivity.
Other important (acidic) retarders/inhibitors include benzoic, salicylic, and phthalic anhydride when used with thiazoles. They not only delay the onset of cure but also slow down the cure rate of vulcanization. It is believed that the cure delay is caused by the formation of a PA-Zn-MBT complex during cure.6 An alternative explanation is "starvation" of zinc oxide by phthalic anhydride. However, this is not very probable because anhydrides are generally ineffective with sulfenamides.6
References and Notes
- P. N. Son, K. E. Andrews, and A. T. Schooley, Rubber Chem. Technol., Vol. 45, No. 6 (1972)
- R. I. Leib, A. B. Sullivan, C. D. Trivette Jr, Rubber Chem. Technol.,Vol. 43, No. 5 (1970)
- P. Ghosh, S. Katare, P. Patkar, J.M. Caruthers, V. Venkatasubramanian, and K.A. Walker, Rubber Chem. Technol., Vol. 76, No. 3, 592-693 (2003)
It is believed that MBTS dissociates on heating to form free radicals. The overall mechanism most likely involves both radical and ionic reactions because it was found that radical scavengers retard the cross-linking reaction.5
- MHS. Gradwell, and WJ. McGill, J. Appl. Poly. Sci., Vol. 61, 1131-1136 (1996)
- P.N. Son, Rubber Chem. and Technol., Vol. 49, no. 1, pp. 118 - 125 (1976)
According to Gradwell et al.8 the reaction of CTP with MBT inhibits further polysulfide formation. Thus, it prevents the formation of MBT and only after all CTP has been consumed does crosslinking continue at normal rate. However, it was also shown that CTP does not delay the onset of crosslinking, i.e. it does not act as a true inhibitor but as a retarder.
- MHS. Gradwell, and N.R. Stephenson, Rubber Chem. and Technol., Vol. 74, no. 1, pp. 44 - 56 (2001)